What topics in Year 11 Chemistry are less important?
Don't spend time revising the wrong topics
Before you read this article, we recommend you checking out ‘HSC - Chemistry: What should I revise from Year 11 Chemistry?’ first.
The transition from Year 11 to Year 12 is short-lived during which, as a student, you may be wondering what topics you should revise before going back to school.
We have compiled a list of topics that we think you should not focus much time revising as they are not closely examined in the HSC Chemistry syllabus.
It is important to keep in mind that the following topics are only less important because they are not explicitly required or examined in Year 12 Chemistry. If you’re about to commence Year 11 Chemistry, this does not mean you should neglect these topics when preparing for preliminary assessments.
Disclaimer: Some schools do include a few of the following topics in Year 12 assessments, particularly concepts from Module 4, if they do not finish teaching it in Year 11.
Module 1 – Properties and Structure of Matter
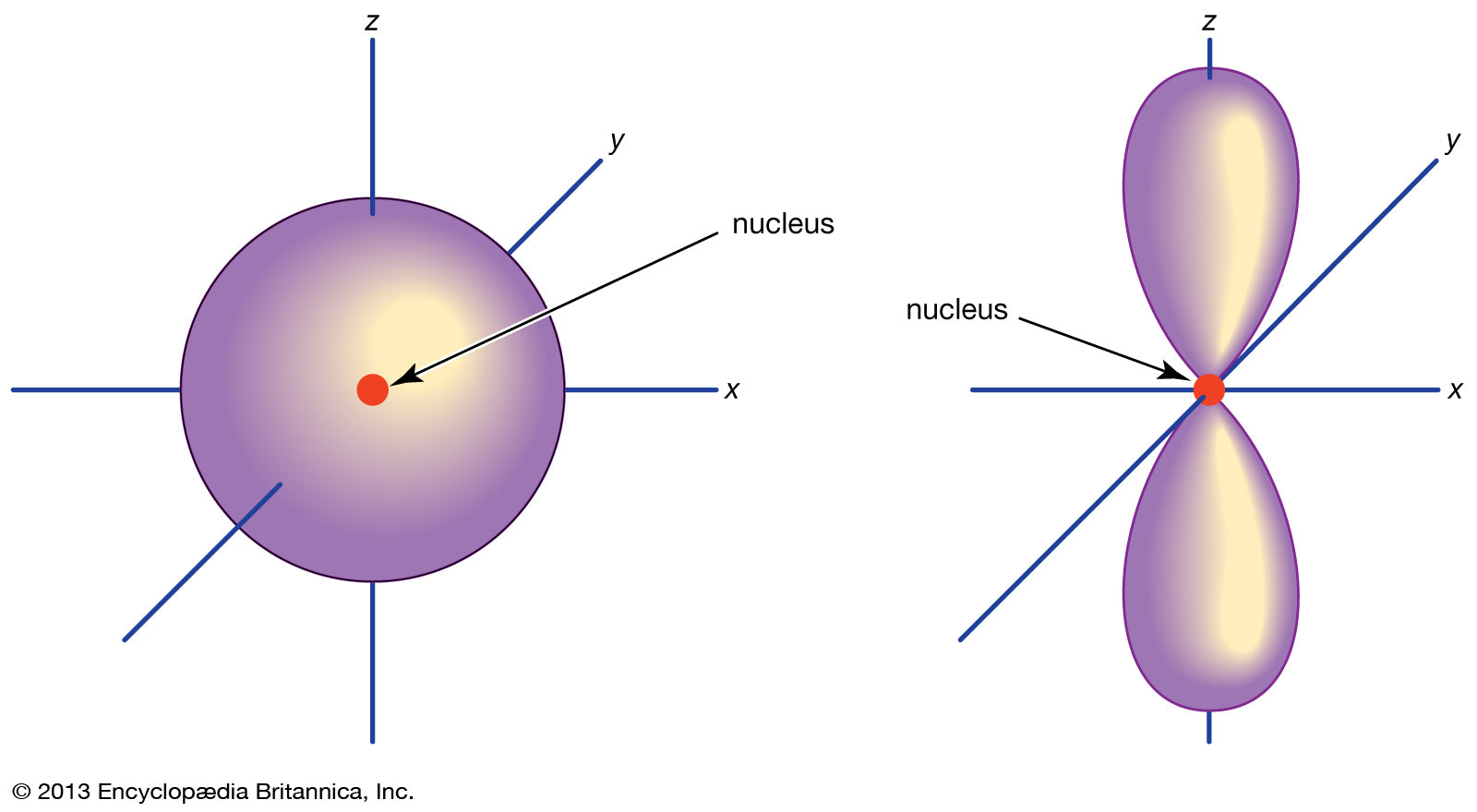
- Spdf configuration and orbitals
This concept won’t be directly and extensively examined in HSC Chemistry.
- Relative atomic mass and isotopic composition calculations
While it is beneficial that you understand what relative atomic mass and isotopic composition are, you will not be asked to perform calculations for them in Year 12.
- Nuclear chemistry: types of radiations and balanced equations thereof.
Module 2 – Introduction to Quantitative Chemistry
- The names of the four gas laws: Gay-Lusaac’s Law, Boyle’s Law, Charles’ Law and Avogadro’s Law.
While it is unlikely that you will be tested on remembering their names, we still recommend understanding how they are applied in calculation. You can strengthen your understanding of these laws through practising calculation problems outlined earlier.
Module 3 – Reactive Chemistry
- Electrochemistry: drawing Galvanic cells, calculating standard cell potentials.
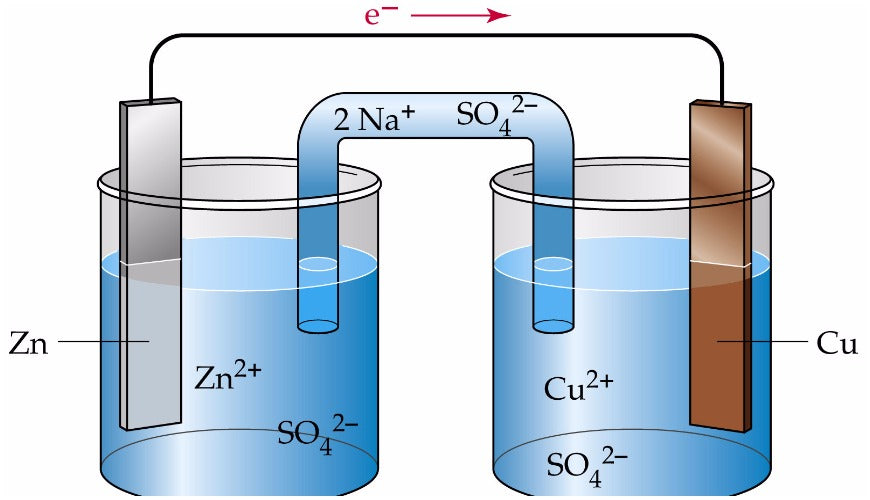
Figure: Galvanic cell created between zinc sulfate, copper sulfate, zinc and copper electrodes.
While most of electrochemistry will unlikely be tested in HSC Chemistry, we recommend students to revise oxidation and reduction reactions as they will be applied indirectly in Year 12.
- Reaction and reactivity of metals with water, dilute acid, oxygen and other metal ions.
Surprisingly, this highly conceptual dot point is not explicitly re-visited in HSC Chemistry. While you won’t need to remember the results of metal reaction experiments, it is still recommended that you understand the underlying principles (see ionisation energy in Module 1).
Module 4 – Drivers of Reactions
As we outlined earlier, some schools may not get a chance to teach or finish teaching this module in Year 11. As a result, students may be tested on Module 4 concepts in their first assessment.
That said, if you have yet to learn or finish learning Module 4: Drivers of Reactions, take the advice below with a grain of salt.
- Enthalpy changes in respiration
While enthalpy changes in respiration are not included in HSC Chemistry, photosynthesis will be re-visited in Module 5: Equilibrium and Acid Reactions.
- Hess’ Law
It is safe to say that Hess’ Law calculations will not be revisited in HSC Chemistry. However, if your school is planning to include this as part of Year 12 assessments, we highly recommend focusing on this when preparing.
Like our content? Subscribe to our newsletter for more free tips and advice.
Let us know what other tips and advice you would like to know in the comment section below!