Intramolecular Bonds and Intermolecular Forces
This is part of Year 11 HSC Chemistry course under the topic of Bonding.
HSC Chemistry Syllabus
- Investigate the role of electronegativity in determining the ionic or covalent nature of bonds between atoms
- Investigate the differences between ionic and covalent compounds through:
- Explore the similarities and differences between the nature of intermolecular and intramolecular bonds and the strength of the forces associated with each, in order to explain the:
Intramolecular Bonds and Intermolecular Forces
Intramolecular Bonds & Polarity
An electric dipole moment is a measure of the polarity of a molecule. These dipole moments occur where there is a separation of electric charge between molecules causing an imbalance. Where this imbalance occurs, we classify molecules as being polar and can use the terms polar and non-polar to describe intramolecular bonds. Whether there is an imbalance of charge present depends on the type of bond and electronegativity of atoms across that bond.
Non-polar Covalent Bond
Non-polar covalent bonds occur where there is an equal sharing or symmetry between the two atoms of the electrons in the bond. Examples of covalent molecules containing non-polar bonds include the diatomic compounds e.g. `Cl_2`, `H_2`, `F_2`, `N_2`, `O_2` where there is no difference in electronegativity of the adjacent atoms. Non-polar covalent bonds are include those with similar electronegativity such as carbon and hydrogen; an example of a molecule that contains such a bond is methane (`CH_4`).
For a covalent bond to be classified as being non-polar, the electronegativity difference between atoms would have to less than 0.5.
Polar Covalent Bond
Polar covalent bonds occur when there is an unequal sharing of the electrons in the bond leading to a charge separation and imbalance within the bond. This occurs when there's a large difference in electronegativity of adjacent atoms (ranging from 0.5 to 2.0).
Examples of molecules with polar bonds include:
- `NH_3` which contains N–H bonds where nitrogen atom is much more electronegative than hydrogen atom
- `H_2O`: which contains O–H bonds where oxygen atom is much more electronegative than hydrogen atom
- `\text{C Cl}_4`: which contains C-Cl bonds where chlorine is more electronegatie than carbon atom
Ionic Bond
While ionic compounds do not exist as molecules, ionic bonds are always classified as polar. Ionic bonds are formed when electrons are completely transferred from one atom to another to form a cation and an anion. This is unlike the sharing of electrons which can be found in covalent bonds. Ionic bonds generally contain elements with an electronegativity difference greater than 2.0.
What Properties Are Affected By Covalent and Ionic Bonds?
Covalent and ionic bonds affect properties of substances that form network or lattice structures (non-molecular structures). This includes ionic compounds (ionic lattices) and covalent network substances (e.g. diamond, silicon dioxide).
In contrast, physical properties of covalent molecular substances are affected by intermolecular forces which are discussed below.
Intermolecular Forces
Covalent and Ionic bonds are the forces which bind atoms together to form molecules and compounds. These bonds are a result of electrostatic attraction effects which exist between atoms that exist within molecules and thus they are called intramolecular bonds.
However the forces which exist between molecules are called intermolecular forces. Intermolecular forces play a crucial role in determining the physical properties of covalent molecular substances, including their boiling points, melting points, and states of matter at various temperatures. In order for a substance to transition from solid to liquid state, or from liquid to gaseous state, energy is required to overcome the intermolecular forces. Stronger intermolecular forces require greater energy to overcome which in turn results in higher melting and boiling points.
Dispersion Forces
Dispersion forces, also known as London dispersion forces or van der Waals forces. These forces exist between all types of molecules, as a result of an uneven distribution in electrons around the nucleus and between neighbouring atoms.

As a result of the uneven movement of electrons, one side of an atom can temporarily be more positive than the other size to create a temporary dipole. However because the electron movement within the molecule is random, the dipole constantly breaks and reforms while simultaneously inducing dipoles in adjoining molecules.
The magnitude of dispersion force correlates with the number of electrons in a molecule. Therefore, the larger the molecule (greater its molecular mass), the stronger the dispersion force between it and neighbouring molecules.
Shape of the molecule also plays a significant role in dispersion force. Linear or elongated molecules can come into closer contact with each other than compact, spherical, or branched molecules, leading to stronger dispersion forces due to the increased surface area for interaction.

In the diagram above, the boiling point of hydrocarbon compounds increases as the size of the molecule increases. Greater dispersion force requires more energy to overcome, accounting for increasing boiling points.
Dipole-dipole Forces & Interactions
Dipole-dipole forces are a type of intermolecular force that occurs between molecules that possess a permanent dipole moment. These forces arise due to the electrostatic attraction between the positive end of one polar molecule and the negative end of another. Thus, dipole-dipole forces are only present between polar molecules.
Since dipole-dipole forces result from permanent dipole moments of molecules, they play a more significant role than dispersion forces (formed from temporary dipoles) in the overall intermolecular force between molecules.
A molecule has a dipole moment when there is an uneven distribution of electron density across its structure, leading to regions of partial positive (δ⁺) and partial negative (δ⁻) charges. This uneven distribution can result from the molecule's geometry and the electronegativity differences between its constituent atoms.

For example, in a water (H₂O) molecule, the oxygen atom is more electronegative than the hydrogen atoms, pulling the shared electrons closer to itself and creating a dipole where the oxygen atom is partially negative, and the hydrogen atoms are partially positive.
Factors Influencing Dipole-Dipole Forces:
-
Magnitude of the Dipole Moment:
The strength of dipole-dipole forces is directly proportional to the magnitude of the dipole moment of the molecules involved. Molecules with larger differences in electronegativity between their constituent atoms generally have stronger dipole moments and, consequently, stronger dipole-dipole interactions.
- Molecular Shape and Polarity: The shape of a molecule determines how its dipole moments vectorially add up. Linear or symmetrical molecules may have their individual bond dipoles cancel out, resulting in a nonpolar molecule with no net dipole-dipole interactions. In contrast, asymmetrical molecules can have a net dipole moment, leading to stronger dipole-dipole forces.
Examples:


Hydrogen Chloride (HCl): HCl is a polar molecule with a significant dipole moment due to the electronegativity difference between hydrogen and chlorine. The positive end of one HCl molecule attracts the negative end of another, leading to dipole-dipole attraction.
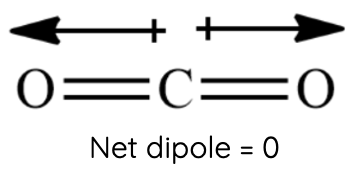
Carbon Dioxide (CO₂): Despite having polar C=O bonds, CO₂ is a linear molecule, and its bond dipoles cancel out, resulting in a nonpolar molecule with no net dipole-dipole forces. However, CO₂ molecules can still experience weaker London dispersion forces.
The significance of dipole-dipole forces is demonstrated by comparing the boiling points of oxygen gas (–183 ºC) and hydrogen chloride (–85 ºC). The two substances have similar molecular mass (therefore similar dispersion force), but the diatomic oxygen gas molecules lack permanent dipoles and cannot form dipole-dipole forces. Dipole-dipole forces between hydrogen chloride molecules require more energy to overcome, accounting for its higher boiling point.
Hydrogen Bonding
Hydrogen bonding is special type of dipole-dipole force, created from the dipole between hydrogen and a very electronegative atom. It is the strongest of the three types of intermolecular forces. Specifically, a hydrogen bond is formed between a partially positive hydrogen atom bound to a fluorine, oxygen or nitrogen atom, and a non-bonding electron pair of either fluorine, oxygen, or nitrogen. The electronegative atom (F, O or N) attracts the electron from the hydrogen atom, creating a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.

Therefore, molecules can form hydrogen bonds if the following two criteria are met:
- a molecule contains a hydrogen atom bound to either fluorine, oxygen or nitrogen atom. This is the donor of the hydrogen bond.
- a molecule contains a fluorine, oxygen or nitrogen atom that has an electron lone pair (non-bonding electrons). This is the acceptor of the hydrogen bond. The fluorine, oxygen or nitrogen atom does not need to be bonded to a hydrogen atom to act as a acceptor.
The significance of the hydrogen bond is demonstrated by comparing the boiling points of hydrogen chloride and hydrogen fluoride; the former cannot form hydrogen bonds whereas the latter can.

HCl, despite having a greater molecular mass (thus greater dispersion force) than HF, has a much lower boiling point. This difference is attributed to the ability of HF to form stronger dipole-dipole forces (due to the greater electronegativity of fluorine) and, more importantly hydrogen bonds.
Intermolecular Force and Physical Properties of Water
Boiling and Melting Points of Water
The boiling point of a substance is significantly influenced by the strength of intermolecular forces that must be overcome to convert the substance from liquid to gas. Water's relatively high boiling point for its molecular size can be attributed to the strong hydrogen bonding between water molecules, which requires a considerable amount of energy to disrupt:
- The extensive hydrogen bonding network in water (every water molecule can form up to 4 hydrogen bonds) creates a cohesive molecular structure in the liquid phase, making it necessary to input a substantial amount of thermal energy to break these bonds and achieve the gaseous state.

- When compared to other hydrides of Group 16 elements (e.g., H₂S, H₂Se, H₂Te), water has a significantly higher boiling point, highlighting the exceptional nature of hydrogen bonding in water.
Density of Water
The density of a substance is determined by the mass of the substance relative to its volume. The anomalous expansion of water upon freezing (which results in ice being less dense than liquid water) is a direct consequence of the structure of hydrogen bonds.
In the liquid state, water molecules are close together but not in a fixed position, allowing them to move freely and pack more densely than in the solid state. As the temperature decreases, water molecules slow down, and hydrogen bonds become more effective, reaching a maximum density at around 4°C.
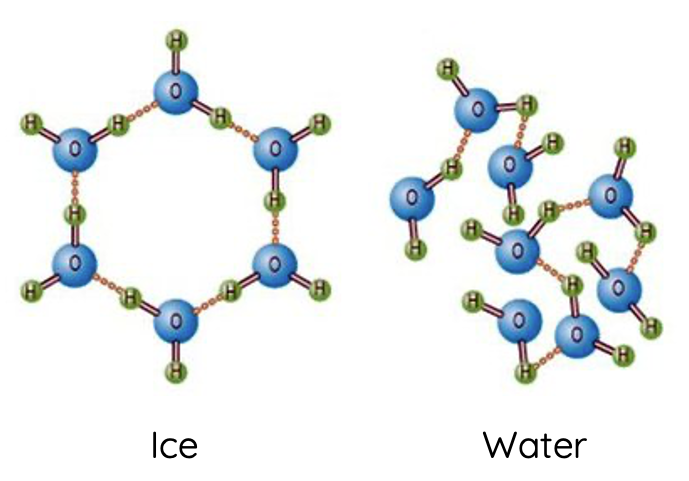
Upon freezing, the hydrogen bonds in water organise themselves into a crystalline lattice that maximises the distance between the water molecules to accommodate the tetrahedral hydrogen bonding geometry. This organised structure takes up more space than the disordered arrangement in the liquid state, leading to a decrease in density.
The lower density of ice compared to liquid water explains why ice floats on water, a phenomenon crucial for the survival of aquatic life in cold climates. The insulating effect of ice helps to maintain a liquid water layer beneath it, providing a habitat for marine life even in freezing conditions.
RETURN TO MODULE 1: PROPERTIES AND STRUCTURE OF MATTER