Lewis Dot Diagram – HSC Chemistry
This is part of Year 11 HSC Chemistry course under the topic of Bonding.
HSC Chemistry Syllabus
- Investigate the differences between ionic and covalent compounds through:
How to Draw Lewis Dot Structure – Master Class
What is Lewis Dot Diagram?
A Lewis dot structure or diagram provides a graphical representation of a molecule's structure. They depict the arrangement of electrons in a compound, and students must learn to construct these structures to comprehend the electronic arrangement within compounds.
Here are the steps to draw a Lewis dot diagram:
1. Determine the total number of valence electrons available for bonding.
- Calculate the total valence electrons available for each atom in the molecule.
- Draw the valence electrons around each atom.
If the compound or molecule is charged e.g. polyatomic ions:
- Add an electron for each negative charge.
- Subtract an electron for each positive charge.
2. Draw a hypothetical structure.
- Arrange the atoms with a central atom (typically the least electronegative) surrounded by outer atoms. Bear in mind that hydrogen is always an outer atom and cannot be a central atom because it only has one electron.
- Assign electron pairs by connecting all atoms to the central atom with single bonds, each representing one shared electron pair. For each bond formed, remove a pair of electrons from a neighbouring atom.
3. Consider and complete the octet rule
- Calculate whether every atom in the molecule has completed its valence electron shell.
- If not, move electrons between non-bonding pairs and bonds in order to satisfy the octet rule for every atom.
- Some atoms can have fewer electrons than the octet rule. For example, atoms in molecules with odd number of valence electrons.
- Some atoms can have more electrons than the octet rule (expanded octet). Examples of these elements are found in period 3 and beyond e.g. sulfur and phosphorus.
4. Calculate and minimise the formal charge on the central atom.
The sum of the formal charges equals the charge of the species. Adjust the number of lone pairs and bonds of each atom to minimise the formal charges of atoms while keeping the sum of formal charges constant.
If the compound has an overall charge e.g. polyatomic ion, draw a pair of brackets around it and label the charge in the top right corner outside the bracket.
Example 1 – Water

Step 1: The total number of valence electrons in a molecule of water `= 6 + 2 xx 1 = 8`.
Step 2: Oxygen atom is the central atom (even though it is more electronegative than hydrogen) because hydrogen atoms cannot be the central atom. Draw single covalent bonds connecting the oxygen atom to the two hydrogen atoms by using valence electrons of the carbon atom.
Step 3: Every atom in the hypothetical structure fulfills the octet rule.
Step 4: The formal charges on oxygen and hydrogen atoms are zero so we this is the correct Lewis dot structure of water.
Example 2 – Carbon Dioxide

Step 1: The total number of valence electrons in a molecule of carbon dioxide `= 4 + 2 xx 6 = 16`.
Step 2: Carbon atom is less electronegative than oxygen, so it is the central atom. Draw single covalent bonds connecting the carbon atom to the two oxygen atoms.
Step 3: The carbon atom only has 4 valence electrons (in two bonds), and therefore needs 4 more electrons to complete its octet. This can be achieved by moving an electron pair from each of the oxygen atoms to form a second bond.
Step 4: The formal charges on carbon atom is `4 - 0 - 4 = 0`, and oxygen atoms is `6 - 4 - 2 = 0`. Since formal charges are zero, this is the Lewis dot structure of carbon dioxide.
Example 3 – Sulfur Trioxide

Step 1: Sulfur is in group 16: 6 valence electrons. Oxygen is in group 16: 6 valence electrons. The total number of valence electrons is `6 + 3 xx 6 = 24`.
Step 2: Sulfur is less electronegative than oxygen, so we expect the sulfur atom to be the central atom, surrounded by three oxygen atoms. Connect the sulfur atom to the three oxygen atoms using single bonds using the valence electrons of the sulfur atom.
Step 3: The sulfur atom only has 6 valence electrons (in 3 bonds) and therefore needs 2 more to complete its octet. This can be achieved by moving an electron lone pair from one of the oxygen atoms to form a second bond.
Step 4: The formal charges of sulfur and oxygen atoms are shown:

In the structure on the left, the formal charge of sulfur `= 6 - 0 - 4 = 2`, and the two oxygen atoms with single bonds `= 6 - 6 - 1 = -1`.
While the formal charges add to an overall charge of zero, we can minimise the formal charges of individual atoms by converting the lone pairs of the other two oxygen atoms to two bonds, such that sulfur will have 6 covalent bonds and 12 electrons (allowed due to sulfur's expanded octet).
Example 4 – Nitrogen Oxide

Step 1: Nitrogen is in group 15: 5 valence electrons. Oxygen is in group 16: 6 valence electrons. The total number of valence electrons is `5 + 6 = 11`.
Step 2: Connect the two atoms via a single bond using the valence electrons of the nitrogen atom.
Step 3: The nitrogen atom only has 5 valence electrons in the hypothetical structure. If we move an electron pair from the oxygen atom to form a second bond, the number of valence electrons of nitrogen increases to 7. If we repeat this process, the number of valence electrons increases to 9. Since the total number of valence electrons is odd (see step 1), we cannot complete the octet rule for nitrogen.
Step 4: The correct Lewis dot structure contains 2 bonds between the nitrogen and oxygen atom because they have zero formal charge.
Example 5 – Hydroxide
Hydroxide ion is an example of an polyatomic ion with an overall charge of –1.
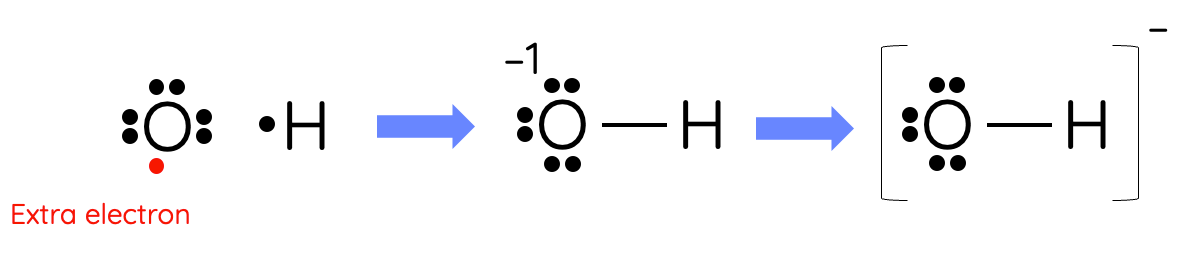
Step 1: The number of valence electrons between oxygen and hydrogen is `1 + 6 = 7`. Since the overall charge of the ion is –1, we need to add an additional electron so the total is now 8 electrons.
Step 2: Connect the two atoms via a single bond using the additional electron we added and the single electron of hydrogen.
Step 3: Both oxygen and hydrogen have completed their octet.
Step 4: The oxygen atom has a formal charge `= 6 - 6 - 1 = -1` which accounts for the overall negative charge of hydroxide. This is the correct Lewis dot structure for hydroxide.
Lewis Dot Diagram for Ionic Compounds
Drawing Lewis dot diagrams for ionic compounds involves representing the transfer of electrons from metal atoms (typically) to nonmetal atoms, resulting in the formation of positively charged cations and negatively charged anions. Unlike covalent compounds, where electrons are shared, ionic compounds involve complete electron transfer.
Since the metal atom in an ionic compound loses its electron(s) to complete its octet, it is drawn without valence electrons. On the other hand, the non-metal atom gains electron(s) to complete in its octet, so it is drawn with a fully occupied valence shell.
In addition, the cation and anion should be labelled with their respective charges outside a square bracket as shown in examples below. The ratio in the empirical formula of the ionic compound should also be included as numbers in front of the square brackets.
For example for sodium chloride (NaCl):
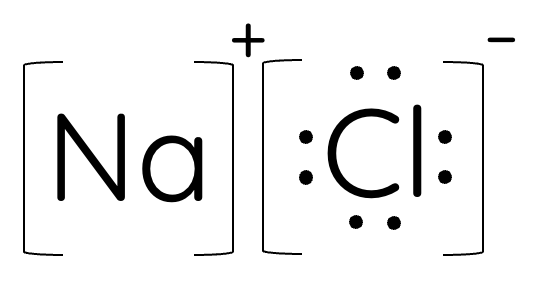
For magnesium fluoride (MgF2):

RETURN TO MODULE 1: PROPERTIES AND STRUCTURE OF MATTER