Identifying Hydroxyl Groups: Lucas' Test, Oxidation Test, Sodium Metal Test
This is part of the HSC Chemistry course under the section: Analysis of Organic Substances.
HSC Chemistry Syllabus
- Conduct qualitative investigations to test for the presence in organic molecules of the following functional groups: hydroxyl groups
Sodium Metal Test
The Sodium Metal Test is a qualitative analytical procedure used to identify the presence of alcohols in a sample. The test exploits the reactive nature of sodium metal when it comes into contact with an alcohol, resulting in the formation of hydrogen gas and an alkoxide salt, according to the reaction:
$$2ROH(l) + 2Na(s) \rightarrow 2RONa(aq) + H_2(g)$$

The reaction between sodium and alcohol involves the breakage of the O-H bond in the alcohol and the formation of a new bond between the alkoxide ion and sodium ion. This process releases hydrogen gas as a byproduct. The ease with which this reaction occurs can vary depending on the structure of the alcohol, with primary alcohols generally reacting more readily than secondary or tertiary alcohols.
Pop-Test
The Pop Test is a complementary procedure used to confirm the production of hydrogen gas during the sodium metal test. When a lit splint is introduced into the test tube containing the gas, the hydrogen reacts exothermically with oxygen in the air to produce water vapor, emitting a distinctive 'pop' sound. This sound is indicative of the presence of hydrogen gas, thereby confirming the presence of an alcohol in the tested sample.
$$2H_2(g) + O_2(g) \rightarrow 2H_2O(l)$$

Advantages and Limitations of Sodium Metal Test
The sodium metal test has both advantages and limitations when it comes to its ability to detect hydroxyl groups.
Advantages:
- The test is simple and safe to conduct under laboratory settings.
- There is no involvement of secondary or side reactions.
- It provides quick results, making it suitable for preliminary testing.
Limitations
- Sodium's reactivity with substances other than alcohol can lead to false positives.
- Test does not provide information on an alcohol's nature (1°, 2 °, 3°), nor does it distinguish alcohols from carboxylic acids, both of which can produce hydrogen gas in the presence of sodium.
- Sometimes sodium can have difficulty reacting with certain alcohols meaning slow reaction with bulkier alcohols may lead to false negatives.
Lucas' Test & Oxidation Tests For Alcohols
This video explores the Lucas test and oxidation tests using acidified dichromate and permanganate solutions to distinguish between primary, secondary and tertiary alcohols.
What are primary, secondary and tertiary alcohols?
Oxidation Test For Alcohols
- Oxidation states of carbon atoms in organic molecules
- Every bond between C and another C does not alter the oxidation state
- Every bond between C and H will decrease the oxidation state by 1
- Every bond between C and a more electronegative element will increase the oxidation state by 1

- The oxidation of a primary (1º) alcohol yields aldehyde as an intermediate and carboxylic acid as the final product.

- The oxidation of a secondary (2º) alcohol produces ketone. Ketone cannot be oxidised further as this would break a C–C bond which requires too much energy.

- Tertiary (3º) alcohols cannot be oxidised because this would otherwise involve breaking a C–C bond (sigma bond) which requires too much energy.

|
Acidified dichromate (Cr2O72–/H+) Oxidation Test
$$\frac{1}{2}Cr_2O_7^{2-} + 7H^+ + 3e^- \rightleftharpoons Cr^{3+} + \frac{7}{2}H_2O$$
|
Observation for different types of alcohol
|
|
Acidified Permanganate (MnO4–/H+) Oxidation Test
$$MnO_4^- + 8H^+ + 5e^- \rightleftharpoons Mn^{2+} + 4H_2O$$
|
|
|
Silver Mirror Test (Tollen’s Reagent)
$$Ag(NH_3)_2^+(aq) + e^- \rightarrow Ag(s) + 2NH_3(aq)$$
|
Tollen’s reagent |
Lucas Test For Alcohols
The Lucas test differentiates between primary, secondary and tertiary alcohols by using Lucas’ reagent: concentrated HCl, ZnCl2 (catalyst).
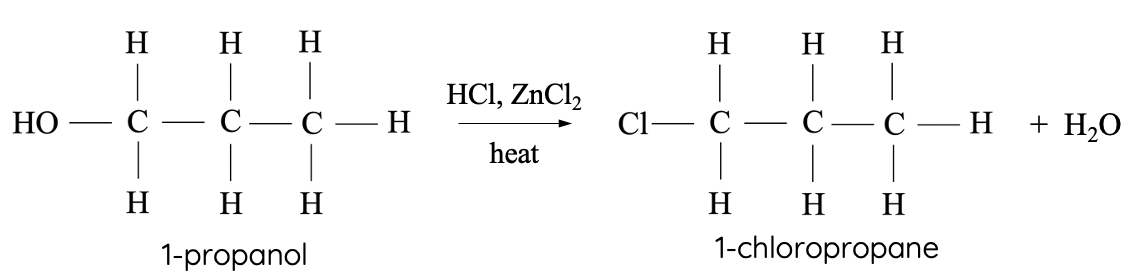
HCl causes substitution reaction with the hydroxyl functional group, resulting in the production of a halogenated hydrocarbon. The halogenated product has lower solubility in water. Thus, the turbidity of solution increases when the substitution reaction takes place. The rate at which the turbidity appears is indicative of the reaction rate.

Image shows a more turbid solution of Lucas’ reagent when a substitution with HCl reaction has occurred.
Observations in Lucas Test:
- Tertiary alcohols react fastest: turbidity quickly appears.
- Secondary alcohols: takes longer time to turn turbid than tertiary alcohols i.e. 3-5 minutes.
- Primary alcohols: no visible reaction at 25ºC. Reaction can be made more apparent with heat.
 Cr6+ (orange) reduces to form Cr3+(green)
Cr6+ (orange) reduces to form Cr3+(green)
 Mn7+ (purple) reduces to form Mn2+ (colourless)
Mn7+ (purple) reduces to form Mn2+ (colourless)