5 Misconceptions You May Have For Organic Chemistry

In this blog post, we will discuss about 5 common misconceptions Year 12 students face when studying the new HSC Chemistry Module 7 syllabus.
Molecular Shape of Organic Compounds
Misconception: Organic compounds are 2D molecules with bond angles fixed at 90º
When students learn to draw the structural formula of organic compounds, it is common to overlook that all molecules are in fact three dimensional.
This is actually one of the major limitations of using structural formulas as models to represent compounds – they don’t provide us with an accurate understanding.
That being said, there are better models chemists use such as those with dashed lines and wedges. The use of dashed lines and wedges helps us visualise the correct orientation of molecules.
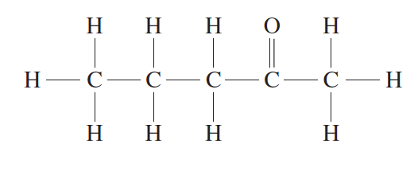
Furthermore, some 2D structural formulas overlook the molecular shape of carbon atoms. For example, a molecule of pentan-2-one depicted below is shown to be linear, with each bond orientated perpendicular to one another.
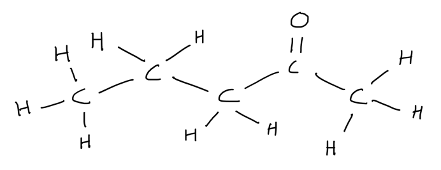
The more correct way to draw pentan-2-one is to follow a ‘zig-zag’ structure, allowing the bonds of the ketal carbon to be placed 120º to one another.
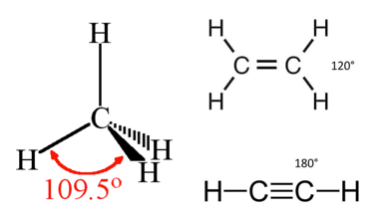
While most structural formulas do not depict molecular shape correctly, students are reminded to know the following:
Angle between bonds of a carbon atom depends on the number of atoms it is bound to:
- 4 bonding partners: tetrahedral shape, angle = 109.5º
- 3 bonding partners: trigonal shape, angle = 120º
- 2 bonding partners: linear shape, angle = 180º
Alkenes vs Alkynes – Reactivity
Misconception: Assuming that since double/triple bonds are stronger than single bonds, they’re less reactive
Misconception arises partly due to a lack of understanding of sigma- and pi-bonds
The investigation of properties of unsaturated carbons is a major component in the hydrocarbons section of HSC Chemistry Module 7 - Organic Chemistry. Previously students would have learnt that double bonds and triple bonds have a much higher bond strength than single bonds.
Students may have the misconception that since the strength of double and triple bonds are much higher than that of single bonds, they must also be much more stable.
What are sigma- and pi-bonds?
Sigma bonds are bonds which are made up of overlapping atomic s orbitals while pi-bonds consist of p-orbitals. Pi-bonds are more reactive than the sigma-bonds and two pi-bonds can be found in triple bonds while only one is found in double bonds and none in single bonds which are made up of only a sigma bond.
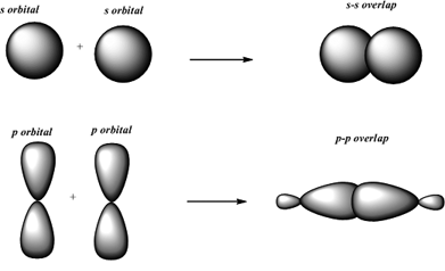
Although both double and triple bonds are stronger, their composition of sigma bonds and pi bonds means that double and triple bonds have a much higher electron density with an increasing number of bonds.
Correct understanding: Double bonds and triple bonds, while being much stronger bonds than single bonds, are immensely more reactive due to their pi-bond composition and higher electron densities.
Alkenes vs Alkynes – Boiling Points
Misconception: Alkynes have lower boiling points than alkenes because they have lower molar mass, thus weaker dispersion forces.This misconception arises quite early on in Module 7 – Organic Chemistry when students are learning about physical properties of hydrocarbons.
While alkenes have lower boiling points than alkanes due to smaller molar mass and weaker dispersion forces, this is not the case for alkynes.
While it is true that alkynes have weaker dispersion forces compared to alkenes, they also exhibit a small degree of dipole-dipole forces due to the presence of highly electron-dense triple carbon-to-carbon bonds.
The movement of these electrons within the bonds cause temporary (partial) charges to be created across the triple bond as shown.
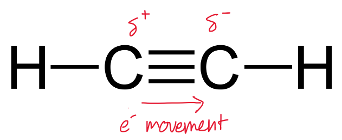
As a result, alkynes actually have an overall stronger intermolecular force than their corresponding alkenes.
Correct understanding: alkynes have slightly higher boiling points than their corresponding alkenes due to small dipole moments created across the electron-rich triple bond.
Flammability vs Volatility
Misconception: volatility is associated with “explosiveness” or a chemical’s flammability.This misconception often occurs towards the end of the hydrocarbons section of Module 7 - Organic Chemistry.
In this section students learn about commonly used hydrocarbons such as Petroleum, Vaseline, Paraffin, Kerosene, etc.
Volatility is a term commonly used to describe these hydrocarbon compounds alongside flammability. While these two terms are frequently discussed together, they do not mean the same thing.
Flammability is the readiness of a chemical to undergo combustion reaction whereas volatility is the readiness of a chemical to transition into vapour from a liquid state.

The symbol on the left is the GHS hazard symbol for flammability but it is often coupled with ‘volatile’ which may cause further confusion for students. Again, volatility and flammability are two different properties but can be both applicable for a given organic compound.
Correct understanding: the volatility of a product is how easily a compound turns from a liquid to a vapour rather than its flammability.
Carboxylic Acid vs Amide – Boiling Points
Misconception: Carboxylic acids have the highest boiling point amongst all functional groups in the HSC syllabus.Carboxylic acid molecules are brought together via dispersion, dipole-dipole forces and most importantly, strong hydrogen bonds. Thus, they have higher boiling points than most functional groups such as alcohols, esters, aldehydes, ketones and of course, hydrocarbons.
However, amides generally have higher boiling points than carboxylic acids with similar molar mass, particularly primary and secondary amides.
This can be explained by two reasons:
- Primary amides have two hydrogen atoms bound to a nitrogen which allows them to form more hydrogen bonds than carboxylic acids.
- Amides have resonance structures which create ionic charges at the carbonyl oxygen atom and amide nitrogen atom. These ionic charges drastically increase the overall attraction between amide molecules.
Resonance Structure Explained
While the concept of resonance structure is not taught in the HSC Chemistry syllabus, it is beneficial to understand it when it comes to amides’ physical properties.
The electrons in the amide functional group are flexible and have the ability to delocalise. Specifically, the pi-bond in C=O can move to the oxygen atom as its lone pair while the lone pair of electrons on the nitrogen can move to form a new pi-bond.
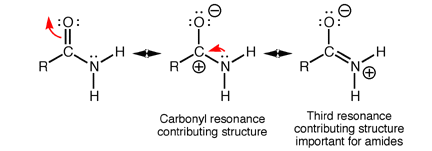
The movement of these mobile electrons generate what’s called a resonance structure as shown above. In the third resonance structure of amide, the oxygen atom is negatively charged while the nitrogen atom is positively charged.
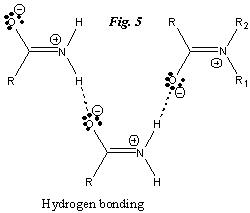
The ‘charging’ of these two atoms strengthen the hydrogen bonds present in primary and secondary amides. In addition, they also increase the attraction between tertiary amide molecules.
What about tertiary amides?
Tertiary amides cannot form hydrogen bonds because they lack hydrogen atoms bound to nitrogen. As a result, the comparison between tertiary amides and carboxylic acids is more difficult. Small tertiary amides have higher boiling point than carboxylic acids whereas large tertiary amides have lower boiling point than carboxylic acids.
Correct understanding: primary and secondary amides have higher boiling points than carboxylic acids with the same molar mass. Small tertiary amides have higher boiling point than carboxylic acids whereas large tertiary amides have lower boiling point than carboxylic acids.
Did you find this helpful?
If so, sign-up to Science Ready to receive new blog post updates.