What Should I Revise From Year 11 HSC Chemistry?
Knowing Everything is Beneficial, but not Essential
Your chemistry teacher may have told you that everything in Year 11 Chemistry is important for HSC Chemistry.
Unfortunately, that's not true. In this HSC Science guide, you will find out why and more importantly, what specific topics you should revise before Year 12 commences.
What you should revise during your school holidays before starting Year 12 HSC Chemistry in Term 4 depends on the teaching order of the Year 12 Chemistry course at your school. The teaching order varies from school to school, so it is important to clarify this before school holiday starts.
Starting Module 5: Equilibrium and Acid Reactions First
Revise:
- Precipitation reactions, collision theory and factors affecting reaction rate from Module 3: Reactive Chemistry.
- Enthalpy, entropy and Gibbs free energy from Module 4: Drivers of Reaction.
Starting Module 6: Acid-base Reactions First
Revise:
- What primary standards are and how to make standard solutions from Module 2
- Chemical reactions involving acids and their chemical equations from Module 3
- Enthalpy change from Module 4
Starting Module 7: Organic Chemistry First
Revise:
- Periodic table basics and its trends from Module 1
- Covalent molecules and intermolecular forces from Module 1
Starting Module 8: Applying Chemical Ideas First
Some schools may start teaching the first inquiry question under Module 8 before starting one of the other three modules.
Revise:
- Precipitation reactions and solubility rules of ionic compounds from Module 3
Module 1 – Properties and Structure of Matter
The first module of the Year 11 Chemistry syllabus explores many fundamental concepts that are pre-requisite knowledge for Year 12 HSC Chemistry. So, unfortunately this means you will need to revise most topics from this Module.
 Figure: brief overview of the four different types of chemical bonding and interactions explored in Year 11 HSC Chemistry course. Image credit: Encyclopedia Britannica
Figure: brief overview of the four different types of chemical bonding and interactions explored in Year 11 HSC Chemistry course. Image credit: Encyclopedia Britannica
Key concepts:
- Understanding of atomic models - most importantly Bohr's model of the atom.
- Naming nomenclature of inorganic substances using IUPAC conventions. This includes understanding the use of valency in naming ionic compounds, as well as the nomenclature of covalent substances.
- Basic structure of the Periodic Table and being able to interpret valuable information from it including atomic number, mass number, electronegativity, ionisation energy and atomic/ionic radii.
- Different types of chemical bonds, intermolecular forces, compounds and their physical properties.
Module 2 – Introduction to Quantitative Chemistry
The theory and skills you've learnt from this module are equally as important as Module 1 because at least 40% of the Year 12 HSC Chemistry syllabus entails calculations e.g. moles, mass, concentrations and volume.
In Module 5: Equilibrium and Acid Reactions, calculations can make up 50%.
If you have struggled with quantitative chemistry in Year 11, we highly recommend you spending more time during school holidays revising these concepts and practising relevant problems to test your understanding and familiarity.
Key concepts:
- Calculation of mass, molar mass, moles, concentration, volume of chemical species in solid, liquid and/or aqueous states. Formulae include:
- Calculations involving dilution of solutions:
$$c_1V_1 = c_2V_2$$
- Calculation of mole, volume, pressure and temperature of gases. Ideal gas equation:
$$PV = nRT$$
- Calculations involving the above concepts with relation to chemical equations and limiting reagents.
Module 3 – Reactive Chemistry
In this module, students learn about different types of chemical reactions, most of which will be explored in greater detail in Year 12 Chemistry. Therefore, it is important to revise these reactions.
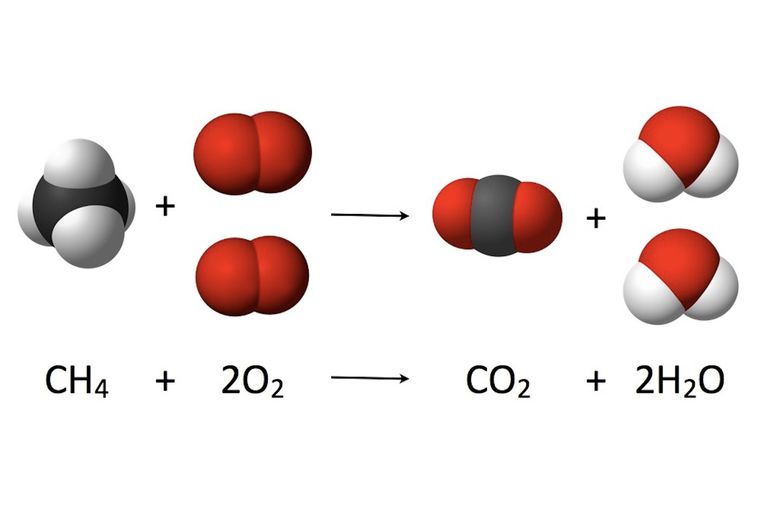
Figure: complete combustion of methane to form carbon dioxide and water.
Key concepts:
- Writing balanced chemical equations. This is perhaps one of the most important skills you will need in HSC Chemistry.
- Different types of reactions including but not limited to:
- combustion (required in module 5 & 7)
- acid-base reactions (required in module 5, 6 and 7)
- precipitation (required in module 5 & 8)
- metal related reactions (required in module 6 and 8)
You should be able to recognise and construct balanced equations for these reactions.
- Concepts regarding catalysts, reaction rate, activation energy and collision theory. These concepts are even more crucial if you are starting with Module 5: Equilibrium and Acid Reactions in Year 12 HSC Chemistry.
Module 4 – Drivers of Reactions
This module is often neglected and sometimes even skipped all together by teachers due to the teaching time constraints in Year 11.
Unfortunately, there are several key concepts in this module you will need to understand in order to excel in HSC Chemistry. This is especially important if you will be start Year 12 Chemistry with Module 5: Equilibrium and Acid Reactions first.
Key concepts:
- Concept of enthalpy and and enthalpy change e.g. endothermic and exothermic reactions. This includes the following topics:
- Energy related to bond formation and breaking
- Enthalpy change of combustion reactions
- Enthalpy change of dissociation of ionic substances in aqueous solution
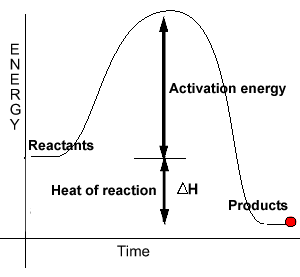
Figure: energy profile of an exothermic reaction.
- Concept of entropy and Gibbs free energy and its relation to enthalpy, entropy and the spontaneity of a chemical reaction. These concepts are important to revise before starting Module 5: Equilibrium and Acid Reactions.
You can find out what topics are less important in the Preliminary HSC Chemistry syllabus here.
Like what you've read? Subscribe to our newsletter for more tips and advice for HSC Science.
What types of tips and advice would you like to see? Let us know in the comment section below!