Aldehydes, Ketones and Carboxylic Acids
This is part of the HSC Chemistry course under the topic Reactions of Organic Acids and Bases.
HSC Chemistry Syllabus
-
investigate the structural formulae, properties and functional group including: – primary, secondary and tertiary alcohols – aldehydes and ketones (ACSCH127) – amines and amides – carboxylic acids
-
explain the properties within and between the homologous series of carboxylic acids, amines and amides with reference to the intermolecular and intramolecular bonding present
-
investigate the differences between an organic acid and organic base
Carbonyl Compounds: Structure, Properties & Reactions
This video introduces a new group of organic compounds – carbonyl compounds, including the structure, properties and reactions of
- aldehydes
- ketones and
- carboxylic acids (organic acid).
Structure and Nomenclature
- Aldehyde, ketone and carboxylic acids all contain a carbonyl carbon that is sp2 This means both functional groups contain a C=O bond, of which one is a reactive π-bond, the other is an unreactive s-bond.
|
Functional group |
Suffix |
Prefix |
Generic structure |
Example |
|
Aldehyde |
-al |
Formyl- |
 |
|
|
Ketone |
-one |
Oxo- |
 |
|
|
Carboxylic acid |
-oic acid |
Carboxyl- |
 |
|
|
Nomenclature priority - In order of decreasing priority: carboxylic acid, aldehyde, ketone, alcohol, alkene, alkyne and alkanes. - In the presence of carboxylic acid, aldehyde or ketone functional group, an alcohol will be referred to by its prefix ‘hydroxyl’
|
 |
Properties of Aldehydes and Ketones
Boiling and Melting Points
- Smaller aldehydes and ketones are polar molecules and there can form dipole-dipole forces on top of dispersion forces.
- Aldehydes and ketones generally have stronger intermolecular forces than hydrocarbons of similar molecular mass. Thus, they have higher boiling and melting points
- Compared to alcohols, aldehydes and ketones generally have weaker intermolecular forces because they cannot form hydrogen bonds, unlike alcohol molecules. Alcohol molecules contain hydroxyl (–OH) groups that can participate in hydrogen bonding as either a donor or acceptor.

- In their own homologous series, boiling and melting points of aldehydes and ketones increase with molecular mass due to stronger dispersion forces.
Solubility in water
|
 |
Table: melting and boiling points of aldehydes and ketones increase with molecular weight (size) while their solubilities decrease with molecular weight.
Properties of Carboxylic Acid
Acidity of Carboxylic Acids
- Carboxylic acids are organic weak acids.
- The deprotonation of hydrogen from a carboxylic acid forms a carboxylate ion.

- The proton or hydrogen atom attached to oxygen is acidic because:
- O–H bond is polarised and weak due to oxygen’s high electronegativity.
- Resonance stabilisation of the conjugate base (carboxylate ion)
- When carboxylic acids are halogenated, the O–H bond becomes more polarised. This means pKa decreases and acidity increases.

- As the carbon chain of carboxylic acids increases in length, acidity decreases and pKa This is because alkyl groups have the opposite effect to that of halogens.

Boiling and Melting Points
|
|
Table: compounds that can form hydrogen bonds have, in general, stronger intermolecular force and higher boiling and melting points than those that do not.
|
Compound |
Functional group |
Molar mass (g mol–1) |
Type of intermolecular force |
Boiling point (ºC) |
|
|
Alkane |
58 |
Dispersion |
–1 |
|
|
Aldehyde |
72 |
Strong dipole |
49 |
|
|
Ketone |
72 |
Stronger dipole |
56 |
|
|
Alcohol |
74 |
Hydrogen bonding |
97
|
|
|
Carboxylic acid |
88 |
Hydrogen bonding |
118 |
Solubility in Water
- Carboxylic acids are more soluble in water than alcohol, aldehydes and ketones of similar molecular weight because they can form more hydrogen bonds.
|
|
Reactions of Aldehydes, Ketones and Carboxylic acids
Oxidation
- Oxidation of alcohols produces aldehyde, ketone and carboxylic according to the following table
|
Reactant |
Reagent/catalyst/condition |
Product |
|
|
Mild oxidising agent
|
|
|
|
Strong oxidising agent
|
Carboxylic acid |
|
|
Any oxidising agent
|
|
- Oxidation of an aldehyde produces a carboxylic acid
Carboxylic Acid and Base Reactions
- Carboxylic acids are weak acids that react with Arrhenius and Brønsted-Lowry bases
- Each carboxylic acid functional group is monoprotic i.e. donates one proton
- Carboxylic acid + metal hydroxide salt + water
Example: reaction between acetic acid (C2H4O2) and sodium hydroxide to produce sodium acetate and water
C2H4O2(aq) + NaOH " NaC2H3O2(aq) + H2O(l)
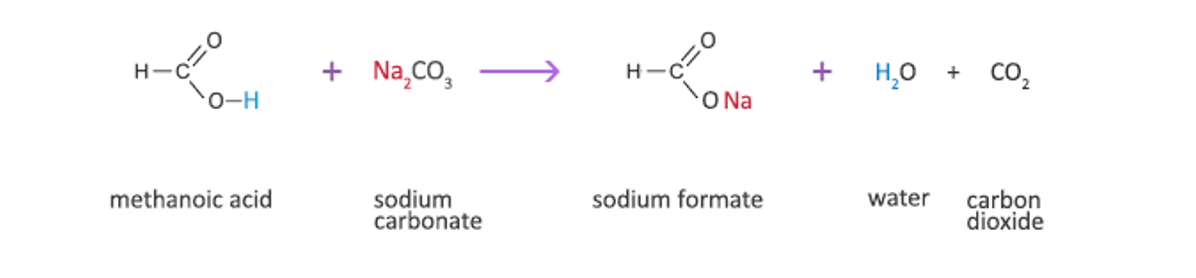
- Carboxylic acid + metal carbonate salt + carbon dioxide and water
Example: reaction between acetic acid (C2H4O2) and sodium carbonate to produce sodium acetate, carbon dioxide and water
2 C2H4O2(aq) + Na2CO3(aq) " 2 NaC2H3O2(aq) + CO2(g) + H2O(l)
- Carboxylic acid + metal hydrogen carbonate salt + carbon dioxide and water
Example: reaction between acetic acid (C2H4O2) and sodium hydrogen carbonate to produce sodium acetate, carbon dioxide and water
C2H4O2(aq) + NaHCO3(aq) " NaC2H3O2(aq) + CO2(g) + H2O(l)
Chemical Tests for Aldehydes, Ketones and Carboxylic acids
|
Dichromate (Cr2O72–) oxidation test
|
Cr6+ (orange) reduces to form Cr3+(green)
|
|
Tollens reagent (silver mirror test)
|
Tollen’s reagent |
|
Permanganate (MnO4–) oxidation test
|
|
Identifying Organic Acids
- This test is used to identify a carboxylic acid from aldehydes and ketones.
- Carboxylic acids undergo acid-base reaction with carbonate and hydrogen carbonates to produce salt, water and carbon dioxide. No acid and base reactions occur between an aldehyde/ketone and carbonates.

CO2(g) + Ca(OH)2(aq) → CaCO3(s) + H2O(l)
Using pH indicators
|
Limewater test of carbon dioxide |






 Hydrogen boding between molecules of carboxylic acids creates a dimer.
Hydrogen boding between molecules of carboxylic acids creates a dimer. Hydrogen bonding between molecules of ethanol does not produce dimers.
Hydrogen bonding between molecules of ethanol does not produce dimers.





 Primary alcohol
Primary alcohol Aldehyde
Aldehyde Primary alcohol
Primary alcohol
 Secondary alcohol
Secondary alcohol Ketone
Ketone


 Mn7+ (purple) reduces to form Mn2+ (colourless)
Mn7+ (purple) reduces to form Mn2+ (colourless)
