Molecular Geometry and VSEPR Theory
This is part of Year 11 HSC Chemistry course under the topic of Bonding.
HSC Chemistry Syllabus
-
Investigate the differences between ionic and covalent compounds through:
– Using nomenclature, valency, and chemical formulae (including Lewis dot diagrams) (ACSCH029)
– Modelling the shapes of molecular substances (ACSCH056, ACSCH057)
What is VSEPR Theory? Identifying Molecular Geometry
VSEPR Theory: The Basics
Developed to predict the shapes of molecules and polyatomic ions, Valence Shell Electron-Pair Repulsion (VSEPR) theory is based on the premise that electron pairs around a central atom will arrange themselves as far apart as possible to minimise repulsion. This theory considers not only bonding electron pairs but also lone pairs of electrons that are not involved in bonding but still contribute to the shape of the molecule.
Key Postulates of VSEPR Theory:
-
Electron Pair Repulsion: Electron pairs around a central atom repel each other. The shape of the molecule adjusts so these pairs are as far apart as possible, minimising repulsion.
-
Lone Pairs and Bonding Pairs: Lone pairs exert a greater repulsive force than bonding pairs, affecting the molecular geometry more significantly.
Predicting Molecular Shapes with VSEPR Theory
The VSEPR theory categorises molecular shapes based on the number of bonding pairs and lone pairs around the central atom.
The following table shows various molecular shapes.
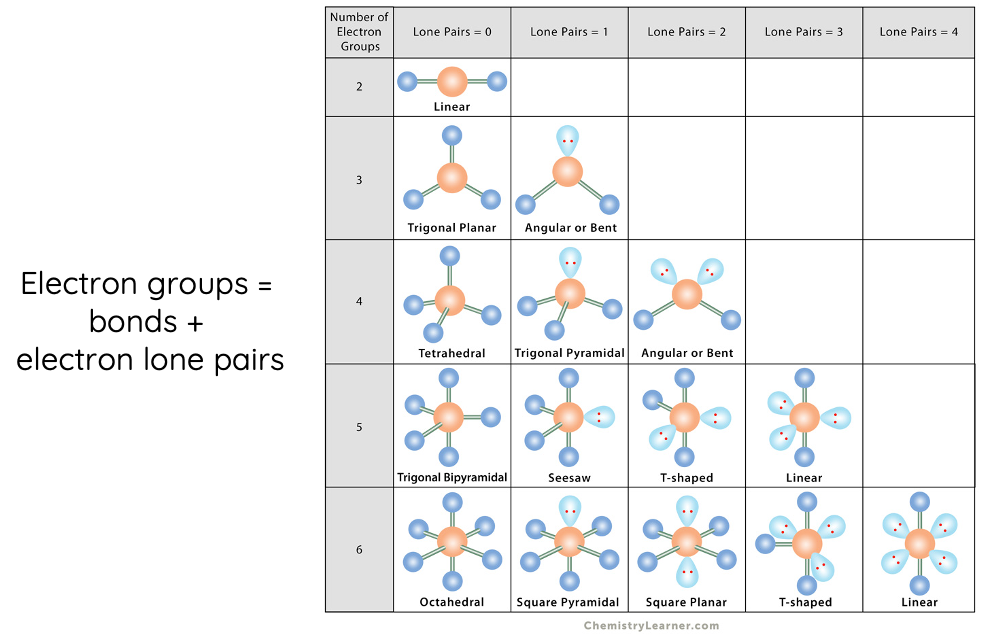
Examples of VSEPR Theory
Example 1 – Beryllium hydride (BeH2)

- Total electron groups = 2
- Bonding groups = 2
- Electron lone pairs = 0
- Shape: Linear
Example 2 – Carbon dioxide (CO2)

- Total electron groups = 2
- Bonding groups = 2
- Electron lone pairs = 0
- Shape: Linear
Example 3 – Boron trifluoride (BF3)

- Total electron groups = 3
- Bonding groups = 3
- Electron lone pairs = 0
- Shape: Trigonal planar
Example 4 – Water (H2O)

- Total electron groups = 4
- Bonding groups = 2
- Electron lone pairs = 2
- Shape: Bent
Example 5 – Methane (CH4)

- Total electron groups = 4
- Bonding groups = 4
- Electron lone pairs = 0
- Shape: Tetrahedral
Example 6 – Ammonia (NH3)

- Total electron groups = 4
- Bonding groups = 3
- Electron lone pairs = 1
- Shape: Trigonal pyramidal
Example 7 – Phosphorus pentachloride (PCl5)

- Total electron groups = 5
- Bonding groups = 5
- Electron lone pairs = 0
- Shape: Trigonal bipyramidal
Limitations of VSEPR Theory
While the VSEPR theory is a valuable tool for predicting the general shapes of molecules and understanding their spatial arrangement, it has several limitations that are important to recognise:
- Lack of Precision in Bond Angles: VSEPR theory can predict the general arrangement of electron pairs around a central atom, leading to the correct prediction of molecular shapes such as tetrahedral, trigonal planar, and linear. However, it often provides only approximate bond angles. The theory does not account for the subtleties that can lead to variations in bond angles due to factors like differences in atom sizes or the presence of multiple bonds.
- Inadequate for Complex Molecules: VSEPR is most effective for simple molecules with a single central atom. It becomes less useful and more cumbersome when applied to large, complex molecules with multiple central atoms or extensive conjugated systems. In such cases, the electron distribution might not follow the simple principles laid out by VSEPR theory, and more sophisticated methods like molecular orbital theory may be needed for accurate predictions.
- Does Not Account for Electron Delocalisation: VSEPR theory treats all electron pairs as localised between specific atoms or as lone pairs on specific atoms. It does not take into account the delocalisation of electrons that can occur in molecules with conjugated pi systems or in aromatic compounds. Electron delocalisation can significantly impact molecular shape and properties, which VSEPR theory does not adequately address.
- Neglect of Molecular Orbital Considerations:The theory does not incorporate molecular orbital theory, which involves the combination of atomic orbitals to form molecular orbitals that can extend over the entire molecule. Molecular orbital theory can provide a more detailed and accurate description of the electronic structure of molecules, especially in explaining phenomena like bond order, magnetism, and the color of compounds, which VSEPR cannot.
- Difficulty with d-Orbital Involvement: VSEPR theory can struggle to accurately predict the shapes of molecules where d-orbitals play a significant role, such as in transition metal complexes. The involvement of d-orbitals in bonding can lead to a wide variety of geometries not easily predicted by the simple electron pair repulsion model.
Note: electron delocalisation and molecular orbitals are beyond the scope of the HSC Chemistry syllabus.