Nomenclature of Inorganic Acids and Bases
HSC Chemistry Syllabus
- investigate the correct IUPAC nomenclature and properties of common inorganic acids and bases (ACSCH067)
How to name inorganic acids and bases
This video discusses the nomenclature (naming convention) of various inorganic acids and bases.
Nomenclature – Naming Acids
Acid names apply to the following two different groups of acids:
- binary acids do not contain oxygen (particularly hydrohalic acids).
- oxoacids (oxyacids) are inorganic compounds made up of oxygen.
Hydrohalic acids
- Hydrohalic acids are aqueous solutions of binary inorganic compounds in which hydrogen, H, is combined with a halogen (Group 17) element.
|
Molecular formular |
Prefix |
+ |
Modified name of element |
+ acid = |
“acid” name |
|
HF |
Hydro |
+ |
Fluorine + ic |
+ acid = |
Hydrofluoric acid |
|
HCl |
Hydro |
+ |
Chlorine + ic |
+ acid = |
Hydrochloric acid |
|
HBr |
Hydro |
+ |
Bromine + ic |
+ acid = |
Hydrobromic acid |
|
HI |
Hydro |
+ |
Iodine + ic |
+ acid = |
Hydroiodic acid |
Oxyacids
- Oxoacids (or oxyacids) are inorganic compounds made up of oxygen (O), hydrogen (H) and one other element (E) called the central atom or central element.
- Examples of molecular formula and their corresponding possible structures showing the general relative positions of hydrogen (H), oxygen (O) and the central element (E) are shown below:
- Oxoacids are named with the name of the central element first using a modified ending (suffix) to indicate the relative amount of oxygen present, followed by the word "acid"

Non-halogenic oxyacids
- The "ic" suffix indicates more oxygen is present in the compound than for the "ous" suffix. The table below includes compounds containing oxygen and hydrogen and one other element that is not a halogen (Group 17) element.
Table: naming nomenclature of oxyacids where the central atom is not a halogen.
|
Central element in oxyacid |
Most oxygen (highest oxidation state) |
Least oxygen (lowest oxidation state) |
|
Nitrogen |
Nitric acid (HNO3) |
Nitrous acid (HNO2) |
|
Phosphorus |
Phosphoric acid (H3PO4) |
Phosphorous acid H3PO3 |
|
Sulfur |
Sulfuric acid (H2SO4) |
Sulfurous acid (H2SO3) |
Nitric acid is a monoprotic oxyacid. One molecule of nitric acid can only donate one proton.
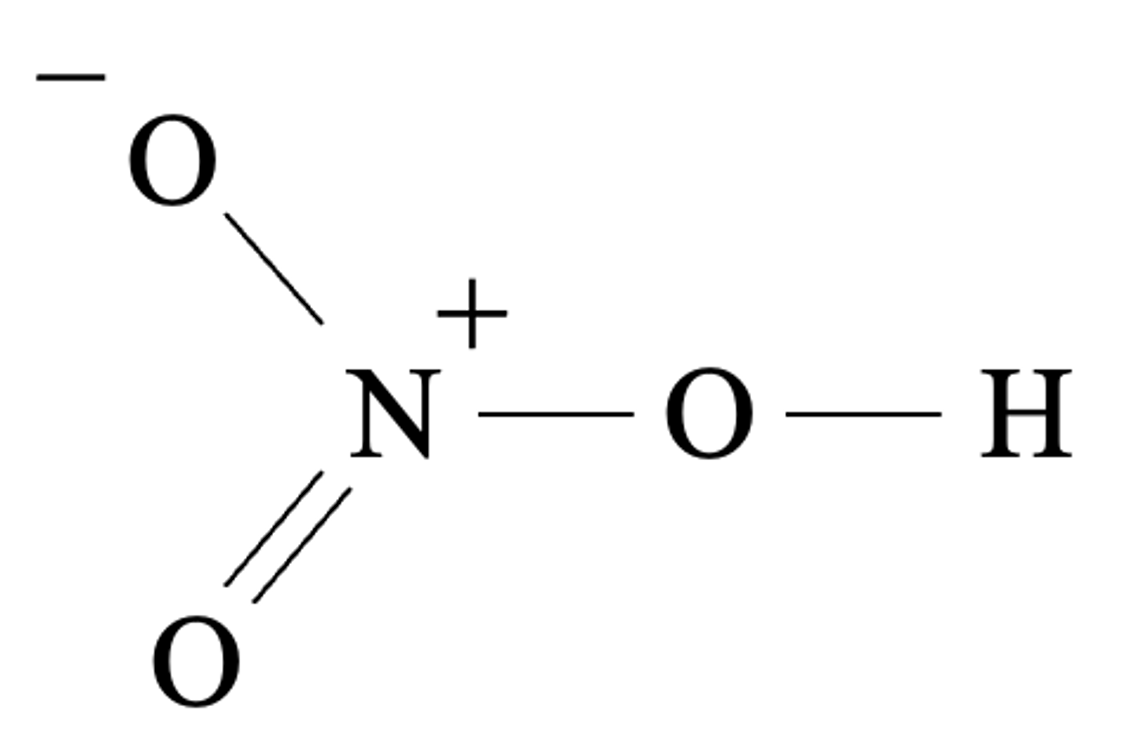
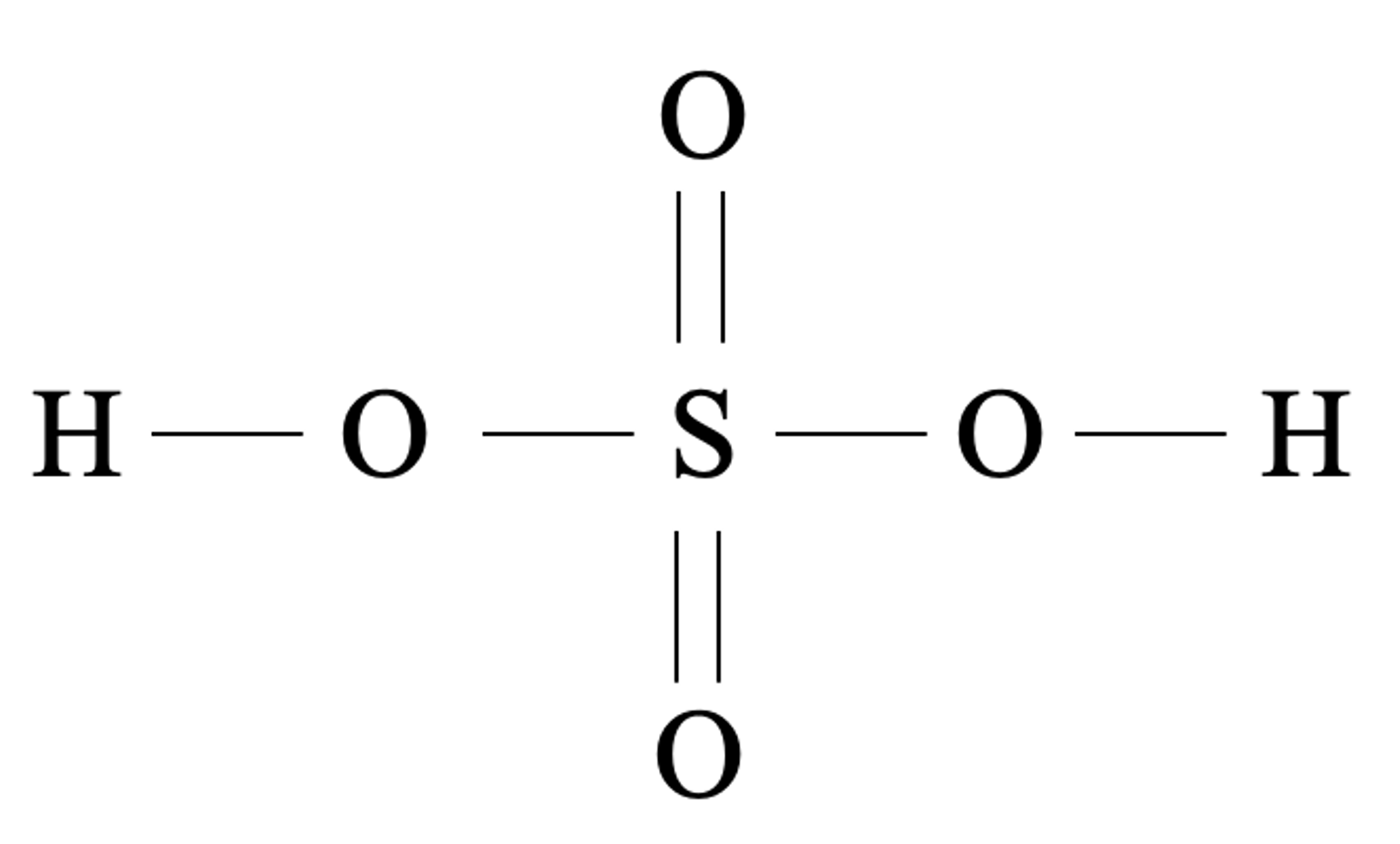
Sulfuric acid is a diprotic oxyacid. One molecule of sulfuric acid can donate up to two protons.
Phosphoric acid is a triprotic oxyacid. One molecule of phosphoric acid can donate up to three proton.

Halogenic oxyacids

- perhalic acid has the most oxygen of all with the general molecular formula HXO4
- halic acid has less oxygen than perhalic acid and has the general molecular formula HXO3
- halous acid has less oxygen than halic acid has the general molecular formula HXO2.
- hypohalous acid has the least oxygen of all and has the general molecular formula HXO
Table: naming nomenclature of oxyacids where the central atom is a halogen.
|
Central element in oxyacid |
More oxygen (highest oxidation state) |
Less oxygen (lowest oxidation state) |
||
|
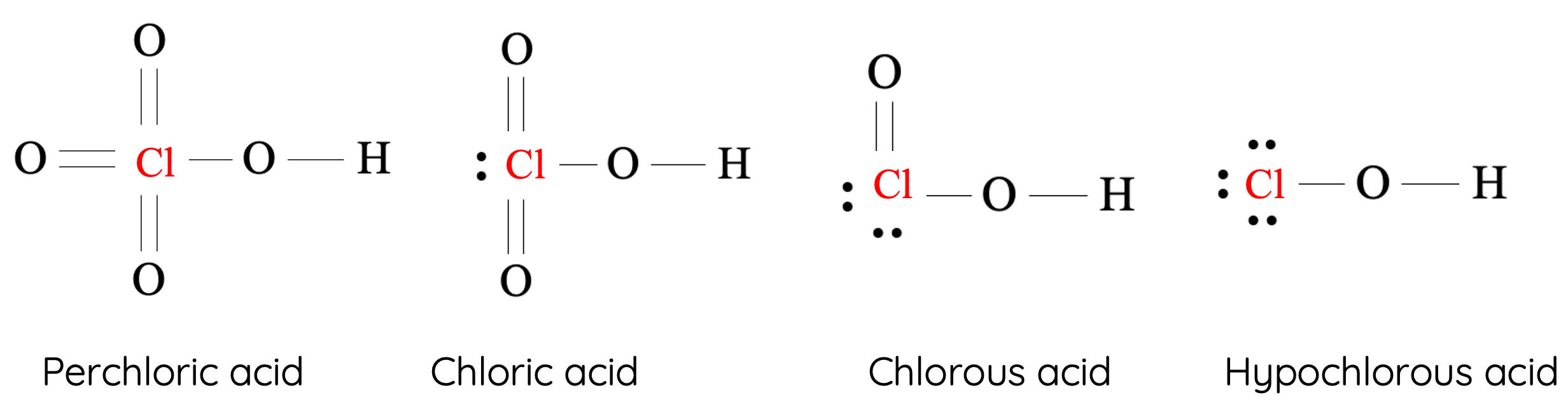
Chlorine |
Perchloric acid (HClO4) |
Chloric acid (HClO3) |
Chlorous acid (HClO2) |
Hypochlorous acid (HClO) |
|
Bromine |
Perbromic acid (HBrO4) |
Bromic acid (HBrO3) |
Bromous acid (HBrO2) |
Hypobromous acid (HBrO) |
For example, the structures of halogenated oxyacid containing chlorine as the central atom are shown:
Nomenclature for Inorganic Bases
The naming convention of inorganic bases follows nomenclature of ionic compounds.
Metal hydroxides:
- NaOH – sodium hydroxide
Non-metal hydroxides:
-
`NH_4OH` – ammonium hydroxide
Metal (hydrogen) carbonates:
- `NaHCO_3` – sodium hydrogen carbonate
- `Na_2CO_3` – sodium carbonate
Common Acids and Bases
Table: common examples of strong and weak acids
|
Strong acids |
Weak acids |
||
|
Molecular formula |
Name |
Molecular formula |
Name |
|
HClO4 |
Perchloric acid |
H3PO4 |
Phosphoric acid |
|
HI |
Iodic acid |
HF |
Hydrofluoric acid |
|
HBr |
Hydrobromic acid |
CH3COOH |
Ethanoic acid (acetic acid) |
|
H2SO4 |
Sulfuric acid |
CH2OOH |
Methanoic aicd |
|
HCl |
Hydrochloric acid |
C6H8O7 |
Citric acid |
|
HNO3 |
Nitric acid |
C2H2O4 |
Oxalic acid |
Table: common examples of strong and weak bases. Note that most weak bases are organic bases (containing carbon).
|
Strong bases |
Weak bases |
||
|
Molecular formula |
Name |
Molecular formula |
Name |
|
NaOH |
Sodium hydroxide |
NH3 |
Ammonia |
|
KOH |
Potassium hydroxide |
NaHCO3 |
Sodium bicarbonate |
|
Ba(OH)2 |
Barium hydroxide |
CH3NH2 |
Methylamine |
|
Ca(OH)2 |
Calcium hydroxide |
(CH3CH2)2NH |
Diethylamine |