Soaps and Detergents
This is part of the HSC Chemistry course under the topic Reactions of Organic Acids and Bases.
HSC Chemistry Syllabus
-
investigate the structure and action of soaps and detergents
Structure and Action of Soaps and Detergents
This video explains the structure and action of soaps and detergents.
Fatty Acids
Fatty acids are organic acids with a carboxylic acid functional group and a with long hydrocarbon chain.
Hydrocarbon chains of fatty acids can either be saturated or unsaturated
- Saturated fatty acids contain only single carbon-carbon bonds
- Unsaturated fatty acids contain double and/or triple bonds.

C=C bonds cause the fatty acid to have a ‘kink’ in its shape. This reduces the effective area of contact between molecules, resulting in unsaturated fatty acids having lower boiling points than their saturated counterparts.
What is Glycerol?
|
Glycerol |
What are Triglycerides?
Triglycerides are large organic molecules that consist of three fatty acid molecules and a glycerol molecule.
Triglycerides are formed from the esterification between glycerol (alcohol) and fatty acids (carboxylic acid). Since a glycerol molecule has three alcohol groups, it will react with up to three fatty acid molecules. Formation of triglycerides is a condensation reaction so three H2O molecules are formed as biproducts.

- Synthesis of triglyceride from fatty acids and glycerol is reversible. The reverse reaction is the hydrolysis of triglyceride which produces glycerol and fatty acids

Saponification
Saponification (production of soaps) is the hydrolysis of triglycerides (fats) using a strong base such as NaOH instead of water.
The presence of a strong base deprotonates what would have been fatty acid molecules to form their conjugate bases (R–COO–). These conjugate base molecules form salts with metal ions from the strong base e.g. Na+ This salt is soap.

Base-drive hydrolysis of triglycerides (saponification)
Water or acid-driven hydrolysis of triglycerides will produce glycerol (alcohol) and fatty acids but will not form soap as protons of fatty acids remain in the molecules in the absence of a base.

Hydrolysis of triglycerides using water
Producing Soaps in the Classroom
A sample experimental method for producing soap from olive oil is outlined below.
- Hydrolysis of olive oil (glyceryl trioleate) using NaOH:

|
Method
|
 |
Note:
- 10 mL ethanol is added as a solvent to help dissolve the oil which is non-polar. The polar part of ethanol will help create a miscible solution of oil and water.
- Dissolving NaOH in water is a highly exothermic process so the solution will become quite hot.
- The use of a water bath or heating mantle will distribute heat more evenly to the reaction mixture.
- After about 30 minutes of heating, the mixture will suddenly become creamy and then start to curdle. This signals that soap has been made.
Structure of Soap

- Soap has a long non-polar hydrocarbon ‘tail’.This part of the molecule is hydrophobic (dislikes water). However, as it is non-polar it will be repelled away from polar substances like water.
- The carboxylate (RCOO–) region of a soap molecule, or the ‘head’, is anionic. This charged region is polar and readily dissolves in water, it is hydrophilic.
How do Soaps Work?
The action of soap can be better understood by examining their structures.
Surface active agents (surfactants) are chemical substances which decrease the surface tension of water. This enables them to emulsify oils so that they can be suspended in water.
Cleaning agents such as soap are surfactants because their hydrophilic heads can dissolve in water while their hydrophobic tails are embeded in the non-polar oil.
This interaction allows an emulsion to form between water and oil. An emulsion
is a stable mixture of two or more liquids that normally cannot be mixed.

Soap molecules help clean grease off surfaces via the following steps:
- Non-polar hydrocarbon tails embed in grease particles, facing away from the water (solvent) because they are hydrophobic.
- Polar, charged hydrophilic carboxylate heads dissolve in water and increase the attraction between water and grease particles.
- When a grease particle is completely surrounded by soap molecules, a micelle is formed. In a micelle, carboxylate heads face away from the grease particle and help suspend the greasy particle in water to form an emulsion.

Properties of Soap
- Chain length of fatty acids determine the final properties of soap. As chain length increases, it becomes less polar and therefore less soluble in water.
- Soap derived from long-chained fatty acids tend to be harder and do not lather as easily as soaps derived from short-chained fatty acids.
- Coconut oil (short-chained) is commonly included as an ingredient in liquid soaps.
- Base used during triglyceride hydrolysis can affect soap properties
- Sodium-based soaps tend to be harder and less soluble in water
- Potassium-based soaps tend to be softer and more soluble in water
- KOH is typically used to make liquid soaps
Disadvantages of Soap
- Soaps are produced from biomass i.e. vegetable oil or animal fats therefore, they are biodegradable
- Soaps cannot be used in acidic solutions: protonation of carboxylate head reduces its hydrophilicity and ability to act as an emulsifier.
- Soaps cannot be used in hard water (high concentration of Ca2+ and Mg2+ ions) as they precipitate with the metal ions to form scum:

What are Detergents?
Detergents are synthetic compounds that mimic the action of soaps.
Disadvantages of soap led to the development of detergents. For example, detergents can be used in acidic solutions and hard water.
The structures of detergents are similar to that of soaps in that they can be divided into
- hydrophilic heads
- hydrophobic tails
The chemical structure of hydrophilic heads determines the type of detergent.
Anionic Detergents
Anionic detergents are detergent molecules that contain negatively charged heads.
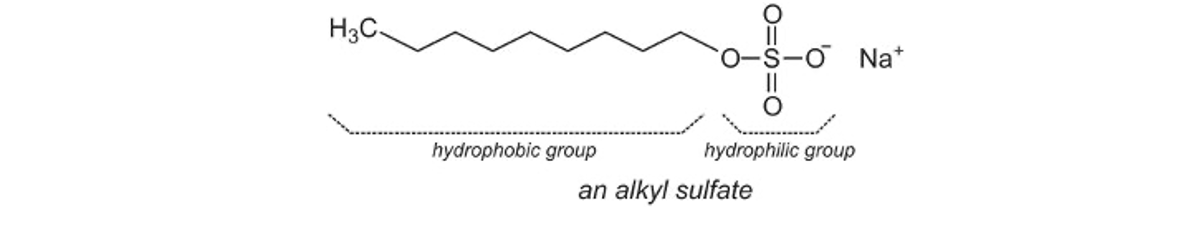
- Long, non-polar tail and an anionic head e.g. sulfate ion (R–SO4–)
- More effective than fat-derived soap as it creates more foam without compromising the cleaning ability of the surfactant.
- Usually not used for personal hygiene as they would remove too much oil from skin and hair.
- Typically found in laundry detergents and dishwashing liquids.
Cationic Detergents
Cationic detergents are detergent molecules that contain positively charged heads.

- Long, non-polar tail and a cationic head e.g. tertiary ammonium ion (R–N(CH3)3+)
- Used in plastic cleaners, hair conditioners and fabric softeners, disinfectants and antiseptics.
Non-ionic Detergents
Non-ionic detergents are detergent molecules that do not contain an ionic charged head. They usually contain heads that are partially charged.

- Long, non-polar tail but a polar head that is not ionically charged. For example, alcohol (hydroxyl) and/or ether (ethoxy) functional groups.
- Typically used in front-loading washing machines and dishwashers
Soaps vs Detergents
Similarities between Soaps and Detergents
- Soaps and detergents share similar structure as their structures consist of a hydrophobic tail and a hydrophilic head.
- Soaps and detergents are both surfactants as their structures allow them to reduce tension between oil and water by forming micelles.
Differences between Soaps and Detergents
- Hydrophilic heads vary between soaps and the three types of detergents.
- Detergents are synthetic whereas soaps are produced from biomass such as vegetable oils and animal fats.
- Although both are cleaning agents, detergents and soaps have different uses. For example, soaps are generally used for personal hygiene whereas detergents are used in laundry, dishwashing and hard surfaces.
Advantages vs Disadvantages
- Detergents form more foam and have better cleaning action than soaps.
- Foam of synthetic detergents are less biodegradable and therefore, impose more environmental implications.
- Soaps cannot be used in hard water as they form scum. This is not an issue with detergents.
- Soaps cannot be used in acidic solutions as protonation of their ‘heads’ reduces their hydrophilicity. This is not an issue with detergents.
