HSC Physics: Spectra of Stars
This is part of the HSC Physics course under the topic Electromagnetic Spectrum.
HSC Physics Syllabus
-
investigate how the spectra of stars can provide information on:
Star's Absorption Spectrum Explained
What is a Stellar Spectrum?
Stars emit radiation which includes visible light (400-700 nm). For example, the Sun emits white light that can be captured and observed on a spectrometer. Instead of seeing a continuous spectrum, the stellar spectra of the Sun contain absent wavelengths (absorption lines) within the spectrum. This is referred to as the absorption spectrum or stellar spectrum.
Surface Temperature
The spectra of stars consist of a wide range of wavelengths. Each wavelength of radiation has a different intensity. The peak wavelength refers to the wavelength with the highest intensity.
We can use this information to compare the relative surface temperature between stars.
Wien’s displacement law quantitatively describes this relationship:

Stars with shorter peak wavelengths have higher surface temperatures.
Table: stars vary in colour, surface temperature and elemental composition. For reference only, in-depth knowledge is not required for Module 7. However, knowledge will be covered and required in Module 8.
|
Spectral Class |
Colour |
Surface temperature (K) |
Elements evident in absorption lines |
|
O |
blue |
over 30 000 |
ionised He, weak H |
|
B |
blue-white |
30 000 – 15 000 |
neutral He, weak H |
|
A |
white |
15 000 – 10 000 |
strong H |
|
F |
white-yellow |
10 000 – 7 000 |
weak H, metals (Ca, Fe) |
|
G |
yellow |
7000 - 5000 |
strong metals, esp. Ca |
|
K |
orange |
5000 - 4000 |
strong metals; CH and CN |
|
M |
red |
4000 - 3000 |
strong molecules (incl. TiO) |
Chemical Composition
Stellar absorption lines are caused by atoms present in gaseous layers of stars.When light is emitted by the star, some of its energy absorbed by atoms in the outer layers of its atmosphere. This occurs when the energy matches exactly the excitation energy of electrons in the ground state of these elements.
The absorption spectral lines represent the energy that has been absorbed by these electrons.

Stars are composed of different elements in the form of atoms and ions. As a result, the pattern of absorption lines of every star's spectrum is unique. As such, the relative position and number of absorption lines can be compared to those of elements on Earth to identify the exact elemental composition of the star.
Motion of the Star
Doppler’s Effect
- The wavelength or frequency of a wave is influenced by the wave’s relatively velocity to the observer.
- If the emitter or source of wave is moving towards the observer, the resultant wave has shorter wavelength and greater frequency.
- If the emitter or source of wave is moving away from the observe, the resultant wave has longer wavelength and lower frequency.
Translational Velocity
Red and blue shifts observed in stellar spectra are caused by the Doppler’s Effect.The following three spectra are for a hydrogen atom.

The movement of stars, whether towards or away from Earth, is demonstrated by red and blue shift effects in their spectra. The shifting effect is apparent when stellar spectra are compared with absorption spectra obtained by exciting elements on Earth.

- When stars move away from us, spectral lines of certain elements e.g. hydrogen would be shifted towards longer wavelengths (red-shift) compared with a reference.
- When stars move towards us, spectral lines of certain elements would be shifted towards shorter wavelengths (blue-shift) compared with a reference.
Rotational Velocity
This effect can also be used to determine the rotational motion of stars.

- Wave emitted from the side of a star that is rotating towards us has shorter wavelength (blue-shifted).
- Wave emitted from the side of a star that is rotating away from us has longer wavelength (red-shifted).
- Wave emitted from the ‘middle’ of a start (region that has no relative rotation to an observer on Earth) has no change in wavelength
The combined effect of these three phenomena results in broadened absorption lines in the stellar spectra.
Density
Width of absorption spectral lines also reveal information about the density of stars.
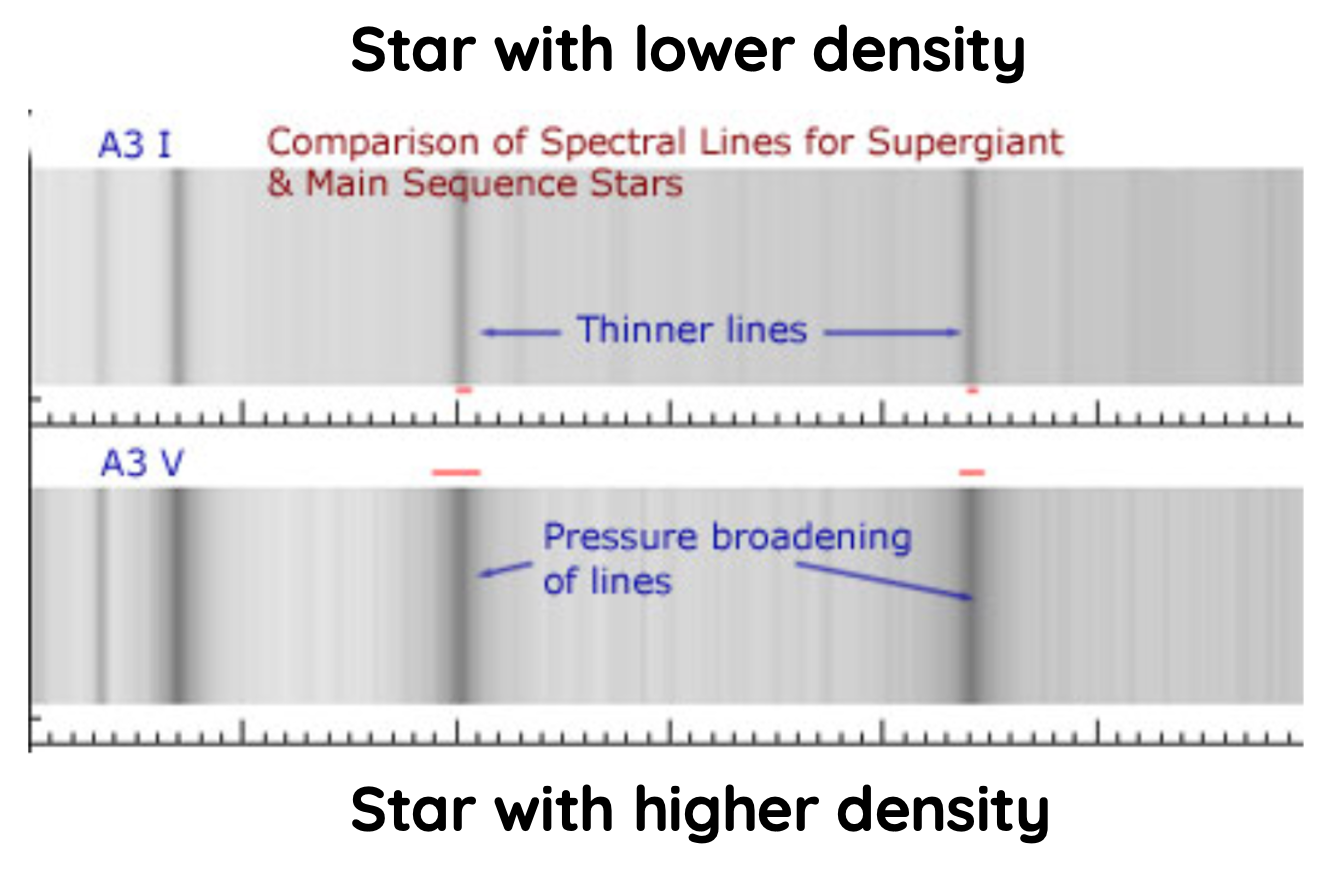
Density and pressure, at the surface of a star can also broaden spectral lines, but the intensity varies across the line in different way from the effect of rotation.
In higher density stars, the increased gas pressure produces more rapid collision between atoms. These collisions result in electrostatic interaction between orbits of different atoms, leading to changes in the energy level of affect orbits.
When the energy state of an orbit increases, the absorption line becomes shorter in wavelength (more energy). When the energy state decreases, the absorption line becomes longer in wavelength (less energy). The combined effect of these changes results in broader absorption lines.
Greater the density of a star, the broader its absorption lines become.
Previous section: Introduction to Spectroscopy
Next section: Diffraction of Light