Spectroscopy – Different Types of Spectra
HSC Physics Syllabus
-
investigate how spectroscopy can be used to provide information about:
– the identification of elements
- conduct an investigation to examine a variety of spectra produced by discharge tubes, reflected sunlight or incandescent filaments
What is Spectroscopy?
Spectroscopy is the interaction between radiation and matter. Specifically, this topic focuses on the interaction between radiation and electrons in matter.

In a Bohr atomic model, electrons orbit the nucleus in stable energy levels. There are multiple energy levels in an atom, the further away an orbit is from the nucleus, the greater the associated energy level it has.
Atomic electrons naturally occupy the lowest energy state known as the ground state. Higher energy states are called excited states.
Electronic Excitation

Electronic Relaxation

Excited electrons do not remain in their excited energy states permanently. When electrons return from their excited state to the ground state, energy is released in the form of electromagnetic radiation (EMR).
During this process, electrons will release the same amount of energy as they absorbed (law of conservation of energy). Since atoms have multiple excitation states, there may be multiple electronic transitions, causing the emitted electromagnetic radiation to vary in energy and thus have different frequencies and wavelengths.
Absorption and Emission Spectrum

Absorption Spectrum
White light consists of visible light of all wavelengths. When white light is dispersed through a glass prism, it forms a continuous spectrum.

When the same source of light is passed through atoms, ground state electrons can absorb specific amounts of energy to transition to higher energy states.
Different energy of light corresponds to different frequencies/wavelengths. Therefore, absorption of light during electronic excitation causes there to be absent frequencies/wavelengths of light in the otherwise continuous spectrum. These absent lines are called absorption lines. This type of spectrum is called an absorption spectrum.
For atoms of a particular element, there are usually more than one absorption line present because a ground state electron can be excited to different excited energy states by absorbing different amounts of energy (in the form of light).
Emission Spectrum
In a sample of hot gas where electrons are in their excited energy states due to the provision of heat as an energy source, these electrons can return to their ground states and release energy in the form of EMR.
The amount of energy released produces EMR of specific frequencies/wavelengths because it equals the difference between energy states. This produces an emission spectrum where only certain frequencies/wavelengths of light are present on a black background. These streaks of light are called emission lines.
Elements have unique emission and absorption spectra.
Emission Spectra of Noble Gases
Emission spectra of noble gases differ in the position and total number of emission lines. Each element produces a unique emission spectrum.

Examples of Various Spectra
Gas Discharge Tubes
Discharge tubes are used to generate atomic emission spectra from gases.
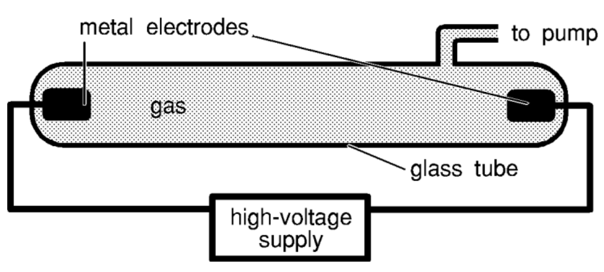
A discharge tube is a simple apparatus consisting of two metal electrodes connected to a high voltage potential difference. The metal electrodes are placed within an enclosed glass tube that contains a particular gas.
Conditions:
- The high voltage (greater than 1000 V) is essential to permit the electric current to pass through air. This is achieved by producing a very strong electric field between the metal electrodes.
- Gas discharge tubes contain low pressure to reduce the movement of gas molecules in air. Reduced movement leads to lower rate of molecular collision such that electrons’ motion (affected by the electric field) are less impeded. Overall, reduced pressure facilitates conduction of current in a gas medium.
When electrons in the current collide with gas molecules in the glass tube, their energy are partially transferred to the electrons in the gas atoms. If the energy is great enough, it will cause excite the electrons. Afterwards, electrons will emit this energy in the form of electromagnetic radiation and return to the ground state.
Depending on the identity of the gas, the emitted radiation may fall in the spectrum of visible light. This means the glass tube will radiate a particular colour.

When the light emitted from a gas discharge tube is dispersed through a glass prism, the light is split into its constituent wavelengths. This produces an emission spectrum.

Dispersion of pink light from a hydrogen gas discharge tube produces an emission spectrum of multiple emission lines. Each emission line corresponds to the energy released for one electronic transition from excited to ground states.
Reflected Sunlight
Reflected sunlight demonstrates absorption spectra of molecules in the atmosphere.
Before reaching Earth's surface, sunlight travels through gaseous layers of the Sun and Earth's atmosphere. Atoms within these layers will absorb light of specific frequencies/wavelengths to form an absorption spectrum.

The gaseous layers of the Sun contains mainly isotopes of hydrogen and helium. The absorption lines on the Sun's spectrum are due to absorption of energy by electrons in hydrogen and helium atoms.

As sunlight passes through Earth's atmosphere, gaseous molecules cause further absorption lines in the Sun's spectrum.
Blue light, due to its higher level of energy compared with other waves of the visible light spectrum, is often absorbed in the atmosphere as the energy is sufficient to excite electrons in various molecules.
Carbon dioxide absorbs infrared radiation and long wavelengths of visible light. Electrons in water molecules also cause absorption of light at various wavelengths.
Incandescent Lamps and Filaments
When electric current is passed through a conductor with very high resistance and melting point (e.g. tungsten), the collision of electrons with the intrinsic structure of the conductor produces heat and light.

In incandescent filaments, the resistance is controlled such that it can generate as much light as possible without completely stopping the movement of the electric current.
Energy transformation: kinetic energy of electron (electrical energy) transformed to heat energy & light energy.
The light produced from an incandescent lamp is unpolarised and forms an continuous spectrum. The spectrum is continuous as it is not a result of electronic excitation but production of actual light. The spectrum contains higher intensity of red light (lower energy) compared with blue light (higher energy)

Previous section: Determination of the Speed of Light
Next section: Applications of Spectroscopy